1) reaction path model


反应途径模拟
1.
The authors compare speciation models with reaction path models, introduce the method of reaction path modelling, give an improvement on the calculation method of pH value in reaction path model.
比较了形态分布模拟和反应途径模拟的差异,总结了反应途径模拟的实现方法,对PH但算法进行了改进,作为实例,模拟了地下水氯上物污染过程。
2) Reaction pathway


反应途径
1.
AM1 method has been used to study the reaction pathway of acrolein in the basis state and the excited states.
用量子化学方法研究了丙烯醛基态和激发态的反应途径,通过比较不同反应途径的反应势垒和中间产物构型的稳定性,从理论上得出该反应的反应机理。
2.
MNDO method has been employed to study reaction pathway a nd to optimizestructure of reactant,product and transition state for the nitro-alhane tautomerism.
用Pulay方法优化了反应物和产物的平衡几何构型,算得了反应途径,获得了过渡态,由虚振动模式对过渡态进行了确证。
3.
NMDO method has been employed to study reaction pathway and to optimizestructure of reactant product and transition state for the dechlorination reaction .
用MNDO方法研究了脱的反应途径和过渡态,论证了O ̄*作为临基参预反应的可行性和优越性。
3) reaction path


反应途径
1.
By searching for the location of the transition states and calculating the reaction heat and the activation energy, it was obtained that the activation barriers of paths 1 and 2 are much low comparatively among four reaction paths, so the major product of the reaction is C 2H 6, which agrees with the experimental result.
提出甲烷等离子体偶联合成碳二烃的主要基元反应是甲烷与甲基、亚甲基、次甲基的自由基反应 ,并用半经验量子化学的 PM3方法 ,对可能的反应途径进行了量子化学研究 ,得到了过渡态的构型 ,计算了反应热及活化能 。
2.
On this basis,the various vibrational frequencies and the normalized eigenvector components for the equilibrium species were calculated,the correlations and changes along the reaction path of various vibrational modes of the reaction system were discussed .
在此基础上,计算了各平衡几何构型不同振动的频率和归一化的本征向量的分量,讨论了反应体系的不同振动模式沿反应途径的相关及其变化。
4) Reaction path simulation


反应路径模拟
1.
Reaction path simulation on Np and Pu in loess-groundwater system;


Np、Pu在黄土地下水系统中的反应路径模拟
2.
This paper discusses the difference of reaction path simulation and mass balance simulation.
反应路径模拟技术是近十几年发展起来的定量研究地下水地球化学演化的重要方法。
5) reaction path and steps


反应途径和步骤
1.
According to conditions of experiment and diagnosed transient components in discharge course of blend flowing of N 2 and H 2 by continuous real time mass spectrometry,the reaction path and steps of synthesizing ammonia were analysed,the standpoint of decay to ammonia via transient component N 2H 6 is presented.
依据试验条件和实时连续质谱诊断的氮氢混合气流放电过程中的瞬态成份 ,分析合成氨的反应途径和步骤 ,提出经由瞬态成份 N2 H6 衰变为氨的观
6) compound of reaction ways


反应途径合成
补充资料:感觉传导途径 3痛觉、温度觉传入途径示意图
李瑞端绘
[图]
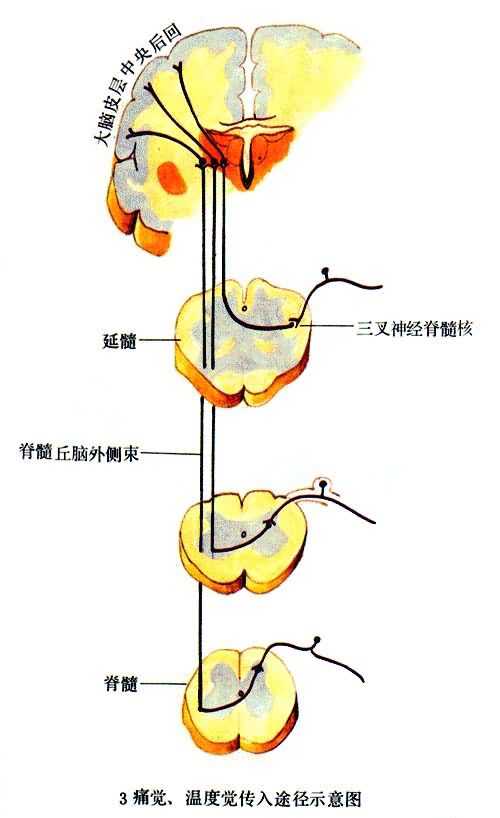
说明:补充资料仅用于学习参考,请勿用于其它任何用途。
参考词条