1) molecular conductivity


摩尔传导率
2) Molar conductivity


摩尔电导率
1.
Modified Relational Model of Molar Conductivity and Concentration;


摩尔电导率与浓度关系模型的探讨
2.
Determination of the infinite dilution molar conductivity of DyCl_3 in isopropanol


DyCl_3在异丙醇中无限稀释摩尔电导率的测定
3.
The use of formula has calculated the molar conductivity of Ce(NO3)3,La(NO3)3 and Y(NO3)3 at 25 ℃.
研究了不同温度下3种稀土硝酸盐:Ce(NO3)3、La(NO3)3、Y(NO3)3在非水溶剂中(无水乙醇、丙酮、DMF)的电导性质,并利用公式求得25℃时Ce(NO3)3、La(NO3)3、Y(NO3)3的摩尔电导率。
3) apparent molar conductivit


表观摩尔电导率
5) Molar conductivity at infinite dilution


无限稀释摩尔电导率
1.
The use of formula has calculated molar conductivity λ of YCl_(3 )in DMF and H_2O, The use of Kohlraush formula has made the diagram push outside and calculated the molar conductivity at infinite dilution λ_(0 )=233.
在293 K时测定了 YCl3 在混合溶剂(DMF H2 O)中的电导率,根据公式求得 YCl3 的摩尔电导率λ值,按Kohlraush经验公式作图,使用Origin软件进行线性拟合外推求得YCl3在混合溶剂(DMF H2O)中的无限稀释摩尔电导率λ0=233 74 s·cm2·mol-1。
2.
15 K to calculate the molar conductivity of DyCl3;secondly,Kohlrausch formula and Origin software were used to make the diagram of linear fitting,finally the diagram was pushed outside and the molar conductivity at infinite dilution(λ0 /(S·cm2 ·mol-1) of DyCl3 in isopropanol was calculated,and the influence of the temperature on λ0/(S·cm2·mol-1) of DyCl3 electrolyte solution was investigated.
15 K温度范围内测定DyCl3在异丙醇溶剂中的电导率,求得DyCl3的摩尔电导率值,应用Kohl-rausch经验规则,使用Origin软件进行线性拟合,作图外推求得DyCl3在异丙醇中的无限稀释摩尔电导率0λ/(S。
6) molar conductivity at infinite clilution


无限稀释摩尔电导率
1.
The use of Origin Software has made the diagram to push outside and calculated the molar conductivity at infinite clilutionλ_0=300.
在298K时测定了NdCl3 在混合溶剂N,N 二甲基甲酰胺水(DMF H2O)中的电导率,利用公式计算了稀土氯化盐NdCl3 的摩尔电导率,使用Origin软件进行线性拟合,外推得到NdCl3 在混合溶剂(DMF H2O)中的无限稀释摩尔电导率λ0=300。
2.
The use of formula Λ_m=(λ_液-λ_剂)×10~(-3)/c has calculated the molar conductivity of NdCl_3 in DMF and H_2O, and using Kohlrausch formula Λ_m=Λ~∞_m(1-βc), The use of Origin Software has made the diagram to push outside and calculated the molar conductivity at infinite clilution λ~∞_m = 300.
在298K时测定了NdCl3在混合溶剂(DMF和H2O)中的电导率,根据Λm=(λ液-λ剂)×10-3/c公式求得NdCl3的摩尔电导率Λm值,应用Kohlrausch经验规则:Λm=Λ∞m(1-βc),使用Origin软件进行线性拟合,作图外推求得NdCl3在混合溶剂(DMF和H2O)中的无限稀释摩尔电导率Λ∞m=300。
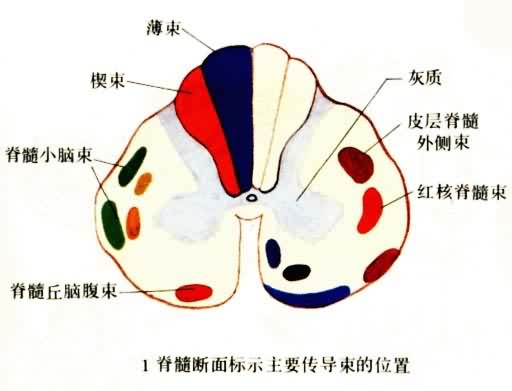
补充资料:感觉传导途径 1脊髓断面标示主要传导束的位置
李瑞端绘
[图]
说明:补充资料仅用于学习参考,请勿用于其它任何用途。
参考词条