1) perfect contract status


合同理想状态
2) assumptive contract status


合同假想状态
3) ideal state


理想状态
1.
It is often Simple in chemistry industry production and chemistry calculation, which just needs to be imagined some ideal state, through which requires pushing at its each level, so that the problem of chemistry industry production has been solved, therefore the ideal result of chemistry calculation is obtained.
化工生产、化学计算往往比较简单 ,需要作一些理想状态假设 ,通过理想状态假设层层推进 ,以解决化工生产的实际问题获得化学计算的理想结
2.
No matter how great the differ ences are,when we understand public opinion itself we should realize it is compl ex and has several levles:public opinion in ideal state refers to the result tha t people understand indepondently information under some conditions.
但无论差异多大,我们在理解“舆论”二字时,都应该认识到它是复杂的、多层次的:理想状态下的舆论指的是在诸多前提条件下的人们对全息信息的独立认识结果;官方控制下的舆论是政治舆论学设想的理想状态,也是现实生活中的常态舆论的主体;民间隐藏的舆论相对于官方控制舆论而言,官方控制越严,民间隐藏的舆论的存在形态和存在方式越多样。
3.
This thesis analyses the accepting obstacles and its reasons, as well as pointing out the ideal state of the accepting process in order to improve moral work and enhance the substantial results of ideological and moral course.
思想品德教育是由传导和接受两个部分构成的,接受的状态和效果,直接影响教学效果,本文通过对思想品德教育的接受障碍及其形成原因的分析,并指出接受过程的理想状态,旨在改进德育工作,增强思想品德课的实效性。
4) contract status


合同状态
1.
Firstly,the engineering contract status was defined and a method of describing the status was proposed,then the contract executing mechanism was revealed on the basis of an analysis of the contract status.
给出了合同状态的定义和合同状态的数学描述方法。
2.
The paper offered the definition and the content of the engineering contract status.


论文阐述了合同状态的含义和内容,通过对合同状态变化本质的研究揭示了合同执行的机理,提出了合同状态的函数表达式。
3.
Secondly,according to the sys-tem\'s law of motion in time,the time-varying process and principle of contract status are clarified and showed by time coordinates.
用集合的形式描述了合同系统的构成要素,阐明了合同状态的时变过程和原理,并且用时间坐标图描述了这一过程,又进一步分析了在FIDIC合同条件下合同状态演进中的矫正规则。
6) idealized state


理想化状态
补充资料:理想气体状态方程
| 理想气体状态方程 ideal gas,equation of state of 描述理想气体状态变化规律的方程。质量为M的理想气体,其状态参量压强p、体积V和绝对温度T之间的函数关系为 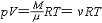 式中μ和v分别是理想气体的摩尔质量和摩尔数;R是气体常量。对于混合理想气体,其压强p是各组成部分的分压强p1、 p2、……之和,故 式中μ和v分别是理想气体的摩尔质量和摩尔数;R是气体常量。对于混合理想气体,其压强p是各组成部分的分压强p1、 p2、……之和,故pV=( p1+ p2+……)V=(v1+v2+……)RT,式中v1、v2、……是各组成部分的摩尔数。 以上两式是理想气体和混合理想气体的状态方程,可由理想气体严格遵循的气体实验定律得出,也可根据理想气体的微观模型,由气体动理论导出。在压强为几个大气压以下时,各种实际气体近似遵循理想气体状态方程,压强越低,符合越好,在压强趋于零的极限下,严格遵循。 |
说明:补充资料仅用于学习参考,请勿用于其它任何用途。
参考词条