1) Solid state Ag/AgCl reference electrode


固态Ag/AgCl参比电极
2) Ag/AgCl reference electrode


Ag/AgCl参比电极
1.
On the basis of the improvement of Ag/AgCl reference electrode of Cortest Company in America, the internal Ag/AgCl reference electrodes were prepared by means of electrolyzing.
在对美国Cortest公司设计的Ag/AgCl参比电极进行改进的基础上,采用电解法制备了一种内置式Ag/AgCl参比电极,对其进行了性能测试,并用其对油管钢N80的高温高压腐蚀行为多次进行了测试研究。
3) solid Ag/AgCl electrode


固态Ag/AgCl电极
1.
Then all-solid Ag/AgCl electrode was made by using the nano-materials,and the morphology was measured by EPMA and SEM.
以此粉体为原料制备了全固态Ag/AgCl电极,采用电子探针和扫描电镜对其表面形貌进行了观察,测试了电极组的自噪声和极差稳定性。
4) novel Ag/AgCl reference electrode


新型Ag/AgCl参比电极
5) Uncovered Ag-AgCl reference electrode


裸露式Ag-AgCl参比电极
6) Ag-AgCl electrode


Ag-AgCl电极
1.
It is the presence of light and hydrogen that increases the Ag-AgCl electrode potential in the physical chemistry experiment.
通过理论分析和实验验证,认为在物理化学实验中Ag-AgCl电极电势的上升主要是光和氢的共同影响。
补充资料:参比电极
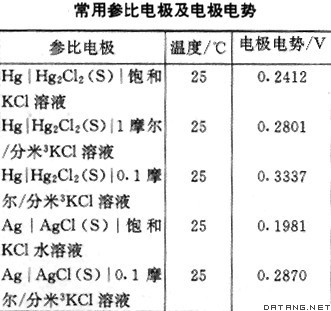
| 参比电极 reference electrode 测量各种电极电势时作为参照比较的电极。将被测定的电极与精确已知电极电势数值的参比电极构成电池,测定电池电动势数值,就可计算出被测定电极的电极电势。参比电极必须是电极反应为单一的可逆反应,电极电势稳定和重现性好。通常多用微溶盐电极作为参比电极,氢电极只是一个理想的但不易于实现的参比电极。常用参比电极列表如下。
|
说明:补充资料仅用于学习参考,请勿用于其它任何用途。
参考词条