1) naphthylamine
[næf'θileimin]


萘胺
1.
Some Factors for Improving Fluorescence Enhancement of β-Cyclodextrin and its Derivatives on Naphthylamine;
影响β-环糊精及其衍生物对萘胺荧光增强效应的一些因素
2.
Assisted elucidation for retention mechanism of naphthylamine and benzenediamine on calix[4]arene stationary phase by quantum chemistry calculation method
量化计算辅助解析杯[4]芳烃固定相对萘胺和苯二胺的保留机制
3.
N,N,N-trimethyl-2-naphthyl quaternary ammonium salt was synthesized from 2-naphthylamine and iodomethane.
以2-萘胺和碘甲烷为原料,首次合成了N,N,N-三甲基-2-萘基季铵盐,其结构经1H NMR,IR和MS表征。
2) 1-Naphthalenamine


a-萘胺
3) naphthalimides


萘酰亚胺
1.
Synthesis and characterization of the derivatives of 1,8-naphthalimides containing thiadiazole heterocycle;
含噻二唑杂环的1,8-萘酰亚胺衍生物的合成与表征
2.
Developments of naphthalimides as anticancer drugs;


萘酰亚胺类化合物作为抗肿瘤药物的研发现状
3.
Synthesis and characterization of some derivatives of 1,8-naphthalimides containing thiazole heterocyclics
一些含噻唑杂环的萘酰亚胺衍生物的合成
4) naphthalimide


萘酰亚胺
1.
Synthesis and Properties of N-Butyl-4-(aza-15-crown-5)-1,8-naphthalimide as a Fluorescent Probe;
4-(氮杂-15-冠-5)-1,8-萘酰亚胺荧光探针的合成及性能研究
2.
Synthesis and Light-rectifying Properties of Novel Naphthalimide Luminescence Dendrimers;
新型树枝功能化萘酰亚胺类发光材料的合成及其光放大效应
3.
Synthesis and solvatochromism of a novel salicylidene schiff base containing naphthalimide;
含萘酰亚胺基元的水杨醛席夫碱的合成及其溶致变色效应
5) α-naphthylamine


α-萘胺
1.
Electrochemical Behaviors of α-Naphthylamine at a Multi-Wall Carbon Nanotube-DHP Film Modified Electrode and Its Application;
α-萘胺在多壁碳纳米管-DHP膜修饰电极上的电化学行为及其测定
2.
Simultaneous Determination of α-Naphthol and α-Naphthylamine by Using Target Factor Analysis-Spectrophotometry;
因子分析-光度法同时测定α-萘酚和α-萘胺
3.
0min,and then the diazo-ion can react with α-naphthylamine to form red azo-compound which could emit fluorescence.
0HAC-NaAC缓冲溶液的条件下,NO2-先与对氨基苯磺酸作用生成重氮离子,后者再同α-萘胺反应生成红色偶氮化合物而发射荧光,Hg2+能催化红色偶氮化合物致使其荧光激烈猝灭,Hg2+的含量与△If成正比的实验事实。
6) 1,1'-bi-2-naphthylamine


联二萘胺
1.
Development in Synthesis of 1,1'-bi-2-naphthylamine and it's Derivative;


联二萘胺及其衍生物的合成进展
补充资料:萘胺
| 萘胺 naphthyl amine 分子式H2NC10H7。有1-萘胺(a-萘胺)和2-萘胺(β-萘胺)两种同分异构体。1-萘胺为无色针状晶体;熔点50℃,沸点300.8℃,相对密度1.1229(25/25℃);微溶于水,溶于乙醇、乙醚;具有不愉快的气味;在空气中逐渐氧化成红色,应避光密封保存。2-萘胺为白色至淡红色叶片状晶体;熔点113℃,沸点306.1℃,相对密度1.0614(98/4℃);溶于热水、乙醇、乙醚;随水蒸气挥发,应避光密封保存。萘胺的萘环上能发生亲电取代反应;萘胺的氨基与亚硝酸作用形成重氮盐,比盐能转变成多种萘的衍生物。 1-萘胺由1-硝基萘还原制得 ;2-萘胺由2-萘酚制备。萘胺主要用作合成染料中间体,本身也曾用作色基。萘胺可经皮肤吸收,生成高铁血红蛋白,造成血液中毒。两种萘胺均为致癌物质,其中2-萘胺是烈性致癌物质,主要导致膀胱癌。 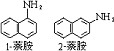 |
说明:补充资料仅用于学习参考,请勿用于其它任何用途。
参考词条